About Hydrogen Induced Cracking (HIC)
HIC ,known as Hydrogen Induced Cracking,
Hydrogen Induced Cracking (HIC) is a common form of wet H2S damage caused by the initiation and propagation of small crack-like flaws, often resembling small internal voids or blisters, that are generally laminar (in-plane) and oriented parallel to the inside and outside surfaces of steel. HIC usually occurs due to the effects of aqueous hydrogen diffusing into steel in wet H2S refinery process environments. It can occur at relatively low temperatures, largely as a result of atomic hydrogen from wet H2S corrosion reactions which enter the steel and collect at inclusions or impurities within the steel. The H2S prevents the hydrogen recombination reaction that would normally occur, so rather than bubbling off from the corroding surface, the hydrogen atoms are forced into the metal structure causing corrosion and weakness.
In the gasoline stabilization distillation column overhead condensers of the oil refining industry, product coolers of hydrodesulfurization units, stripper column overhead condensers, and oilfield gathering and transportation pipelines, etc., carbon steel and low-alloy steel are exposed to hydrogen sulfide-containing environments. Hydrogen generated by corrosion invades the steel and accumulates locally, causing step-like cracking in the steel rolling direction, which is called hydrogen-induced cracking (HIC), and can also manifest as blistering. Hydrogen sulfide is one of the most corrosive harmful media in petroleum and natural gas. During natural gas transportation, hydrogen sulfide accounts for a large proportion of stress corrosion in transportation pipelines. When used in wet hydrogen sulfide environments, hydrogen sulfide can cause hydrogen blistering (HB), hydrogen-induced cracking (HIC), and stress-oriented hydrogen-induced cracking (SOHIC) inside carbon steel. In acidic environments containing hydrogen sulfide and other substances, cracks generated in steel pipes due to hydrogen intrusion from corrosion are called hydrogen-induced cracking (HIC).
Mechanism of Hydrogen-Induced Cracking: When steel is immersed in a hydrogen sulfide-containing environment, hydrogen produced by corrosion penetrates into the steel. Atomic hydrogen diffuses to interfaces such as non-metallic inclusions and transforms into molecular hydrogen at defect sites, increasing the internal pressure of voids.
Hydrogen Corrosion: Hydrogen reacts with carbides in the steel to produce methane. As methane gas cannot diffuse out of the steel, it accumulates between grains to form local high pressure, causing stress concentration and further generating micro-cracks or blisters in the steel.
Hydrogen Embrittlement: Hydrogen atoms generated under various conditions directly penetrate into the steel, reducing the intergranular atomic bonding force, resulting in decreased ductility, percentage reduction of area, and changes in strength. The hydrogen embrittlement theory states that cathodic reactions corresponding to anodic reactions occur at the crack tip. The generated hydrogen or processing-induced hydrogen enters the steel and causes hydrogen-induced cracking.
NACE TM0284
NACE TM0284 is the Test Method Evaluation of Pipeline and Pressure Vessel Steels for Resistance to Hydrogen-Induced Cracking.
Sample Preparation Requirements for Hydrogen-Induced Cracking (HIC)
Sample Dimensions: 100 mm (length) × 20 mm (width).
- Thickness < 30 mm:
- Sampling direction: Parallel to the rolling direction.
- One set of samples per product, with 3 samples per set.
- 30 mm ≤ Thickness < 88 mm:
- Sampling method: Stepwise sampling (through-thickness layered sampling).
- One set of samples per product, with 3 samples per set.
- Thickness ≥ 88 mm:
- Sampling method: Stepwise sampling.
- At least one sample with thickness < 30 mm; the maximum number of samples is taken according to the actual thickness.
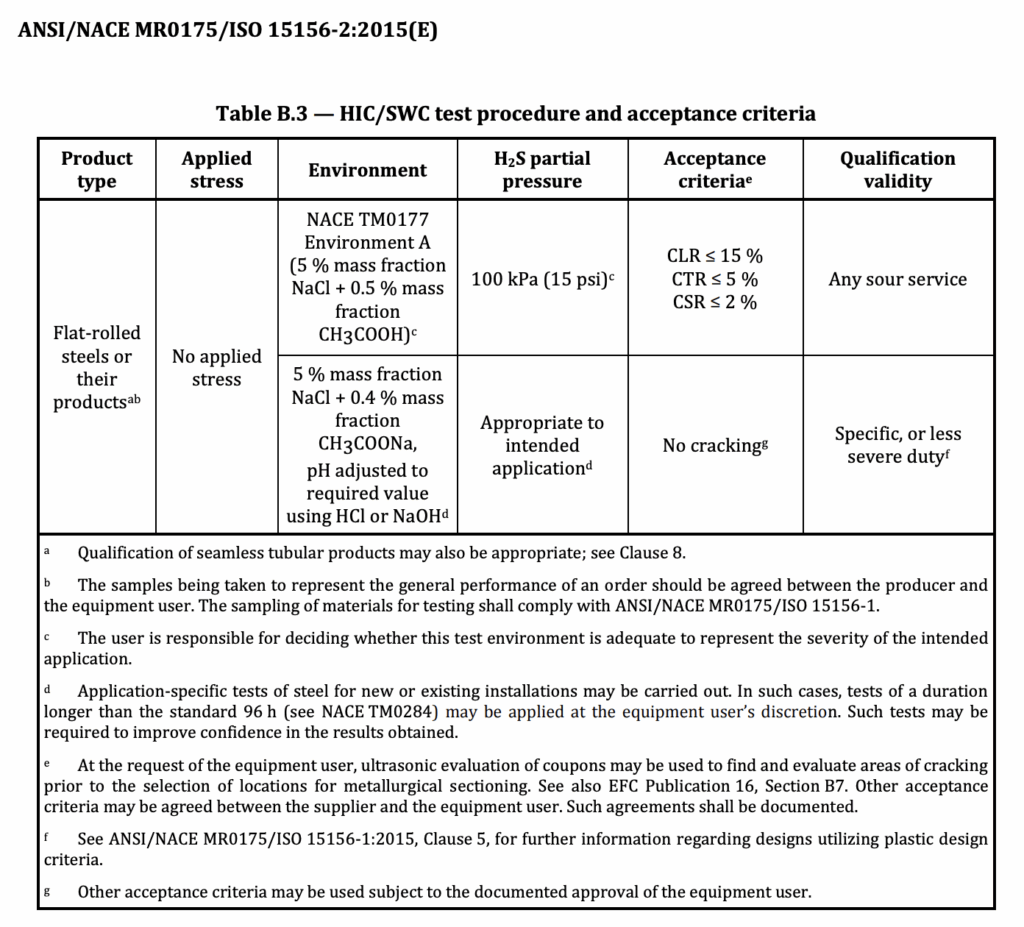
HIC Test Prodcedure and Acceptance Criteria according to NACE MR 0175